
Fundamentally, all batch plants process defined quantities of raw materials to produce the specific quantity, ie, a batch, of finished product. How each plant does this depends on many things including the complexity of the process actions, the number of products the plant must produce, the differences between each product’s process actions and sequences, the level of automation employed and the requirements for batch history.
In many ways, all batch plants are the same, just as people are fundamentally the same. But if we take a closer, in-depth look at the batch plant we find many unique characteristics and requirements that give each plant its own personality. Knowing the personality of a batch plant is the key to selecting the right batch automation solution that best fits the plant's requirements.
Every batch plant has certain basic capabilities that are common to all batch plants. These include the process equipment; equipment control systems; operators who interact with the process; product recipes; a production schedule; and a historical data record of what actually happened.
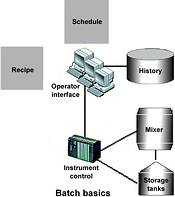
Process equipment
Every plant must have batch processing equipment to hold and process the raw ingredients during production of a batch. The most common equipment types are blenders or mixers.
Equipment control systems
Control of process equipment comes in many forms and is usually dependent on the complexity of the process and the procedure that must be executed to produce that batch. The most basic is a fixed speed mixer motor, with a starter and a timer that is set and controlled by an operator. More advanced process control solutions include PLCs, distributed control systems, PID loop controller and some form of operator interface.
Operator interface
Operators must have a way to monitor and interact with the process. Operator interfaces (OI) can be as simple as a push-button panel or sophisticated, making use of a human-machine interface (HMI) product, like InTouch from Wonderware. The OI serves many purposes, including process visualisation, interfacing to batch and unit control, alarm annunciation and basic recipe management.
Product recipes
Every batch plant has recipes for the products it produces. Every product recipe consists of four common parts: header, equipment requirements, formula and procedure. Let us look at a recipe example, such as a cake mix purchased at your local food store.
The header identifies the product, eg, 'Chocolate cake'. The equipment requirements identifies the required processing equipment.
For the cake mix example, the equipment requirements are detailed on the back on the box: 1 large bowl, mixer, 22 x 30 cm cake pan.
The formula defines the ingredients, such as: 2 eggs, 1 cup water, 1/4 cup vegetable oil, 1 box chocolate cake mix. The procedure defines the processing actions and their sequence of execution, such as: preheat oven to 350°. Combine eggs with water and oil until well mixed. Add cake mix and blend until smooth. Pour into cake pan and bake in oven for 40 minutes.
Every product produced in a batch plant has a recipe with these four parts. They may not be obvious, but they do exist. For example, a recipe may be on a piece of paper that the operator uses to produce the batch. The recipe header and formula are defined on the paper. The equipment requirements and procedure are implied, eg, blend all ingredients together in a blender.
In a more automated process that has a control system, the formula values and procedure may be fixed in that control system. In some plants, the products to be produced all have the same procedure and only the formula changes. If there are only a few products, then formulae can be stored in the control system and the operator selects the product to be run by pushing a button or turning a selector switch to the desired product. Control system logic is used to switch-in the new formula values.
When there are more products than can be stored in the control system, the capability to create, edit and manage recipe formulae is provided externally. Similarly, a facility that downloads new formulae to the control system may be provided when needed.
Production schedule
Plant operations are based on production schedules. The schedule may come from a planning entity somewhere in the company and it is usually produced on a regular basis such as monthly, weekly or daily. The schedule may be produced in different forms, such as a computer printout, a spreadsheet file or electronically downloaded to the batch execution system.
The production schedule received from planning typically only addresses the production of products, not the other collateral production procedures that must be executed, such as the cleaning procedures that are commonly required in the food and pharmaceutical industries.
History
The batch plant must capture all data related to what has been produced in each batch. This may be done in both hardcopy form and electronic archives. The most demanding history requirements are found in the pharmaceutical industry, where all batch events that occurred during production must be captured, including all procedural events such as batch start, hold, restart and complete, recipe procedure execution events, process alarms, operator changes, operator comments, material consumption, material production, and trends related to key process variables.
Complex personality traits
What makes the automation of batch processes so challenging? Obviously, automation of a single-product batch process can be quite straightforward. But when new requirements are introduced, the level of complexity increases dramatically. There are four significant requirements that have notable effects on the complexity of a batch automation solution: the number of products to be made; the procedural changes involved from product to product; the need to produce multiple batches concurrently; and the complexity of each product's historical data.
Number of products
As the number of products to be produced increases, the greater the need for recipe management and for flexibility in the control system logic. A process that will only be making a few products may not need a recipe management system, but a process that produces 20 or more must have some external facility to manage the multiple recipes.
A good recipe management system is important when new product introductions are common or if product recipes are constantly being changed to enhance existing products. Modifying control system code to accommodate the changes can be time consuming and expensive, so it should be avoided wherever possible.
Product procedures changes
When every product has a different procedure, process complexity increases dramatically. One solution that has been used is to change the control system logic for each product. This might be practical if product changeovers are infrequent, such as in long product runs. But if many product changeovers are the norm, such as for making products to customer order, then a sophisticated capability for downloading and editing recipes and procedures must be used.
Additionally, control system logic must be modular in nature and the solution must accommodate a sequencing engine that is easily re-configured for the product being produced.
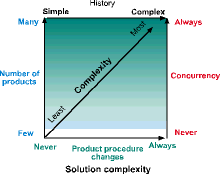
Concurrency
Concurrency is the requirement to produce many different products at the same time on the same production line. In the simplest plant, only one product is produced on a production line at any one time. In a more complex batch process, one process unit is being cleaned, the downstream unit may be finishing production of product X and the upstream may already be producing product Y. Keeping track of what product is in a process unit and when one unit can transfer to another unit can be a daunting task for operators. The automation solution in this situation must employ batch management and equipment arbitration rules to prevent equipment conflicts and potential product contamination.
History
In any batch production process, it is important to have an accurate and complete batch history for many reasons. Proving to oneself (as well as the regulatory agencies) that a drug was manufactured correctly is one driving factor for pharmaceutical companies. In the USA, the drive for electronic batch records has produced the FDA 21CFR Part 11 Regulation on Electronic Records and Electronic Signatures that allows batch history to be recorded electronically.
This is helping to replace older hard copy records and signatures, which were previously the only FDA-acceptable batch records. Optimising the plant is another important reason for capturing batch history. The first step to improving the performance of any process is understanding what the process is doing - and collecting a comprehensive history that captures all events facilitates this enhancement. Hard copy is still acceptable for batch records with simple batch processes and in situations where a complete history is not a requirement. But when product procedures are complex and extensive, filling in the hardcopy batch record can be a time-consuming, inefficient and often error-prone approach.
Identifying and understanding the dominant 'personality traits' of the batch plant is critically important to selecting the most appropriate automation solution.
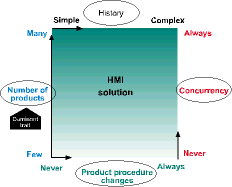
An HMI solution
When the only dominant personality trait is the number of products to be made, then an HMI solution that employs a basic recipe management system for managing product formulae is appropriate - such as InTouch with Recipe Manager. If any other trait becomes dominant, an additional investment in custom software may be required, either in the control system or the HMI.
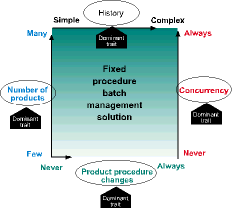
Fixed procedure batch management solution
Concurrency is the dominant trait when there are no product procedure variations; when history is important; and here are many product recipes
As mentioned earlier, concurrency requires sophisticated equipment management logic for equipment arbitration, allocation and release. Only one batch is allowed to own (allocate) a process unit at any given time. Once a batch is transferred to a downstream unit, the upstream unit is released, allowing another batch to allocate the unit for its use.
The most appropriate solution when concurrency is dominant, but recipe procedures are fixed, is one that provides a facility for managing recipes consisting of unit recipes; equipment management logic for concurrency; and automatic capture of batch history.
The Wonderware InBatch FlexFormula Edition product provides these capabilities.
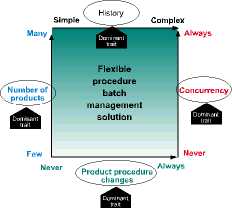
Flexible procedure batch management solution
When all traits are present to the fullest degree, then product procedure changes becomes the dominant trait. As noted earlier, the combination of all four traits brings with it a level of complexity that makes custom solutions very expensive and time consuming to implement and maintain. Advanced batch and recipe management software, like InBatch Premier Edition, have significantly reduced the amount of custom code that needs to be developed. This provides a configuration environment for creation of a control and information model (the process model) that enables plant equipment to be used as defined in the product recipe. The heart of this type of solution is the batch engine, which is responsible for execution of the product recipe, equipment management and batch history.
The right tool for the job
A tool is nothing more than a something that makes a job easier to perform and improves the efficiency of the user. Products like InTouch and InBatch are tools that make batch automation projects easier (assuming the tool is appropriate to the requirements of the project). Understanding the requirements of a batch automation project means understanding the personality of the batch plant and its dominant personality traits so that users can interact with it on a higher level and with the proper tools for the job.

For more information contact Mike le Plastrier, Futuristix Advanced Control Systems, 011 723 9900, [email protected], www.futuristix.co.za
© Technews Publishing (Pty) Ltd | All Rights Reserved