
The measurement of pH is used in many industrial processes some of which are extremely aggressive and can shorten sensor life. Many different types of sensor have been developed to improve performance and increase operational life. An understanding of sensor construction should help the process engineer select the most appropriate type for a given process.
All pH sensors are constructed from two components: a measuring electrode and a reference electrode. They are usually combined into a single sensor referred to as a combination electrode (Figure 1). For the majority of applications, a pH sensitive glass is used for the measuring electrode. This has a high chemical resistance, will withstand temperatures up to ~145°C and can be constructed to operate at very high pressures. Often it is the mounting hardware and seals that limit the operating parameters of the glass electrode.
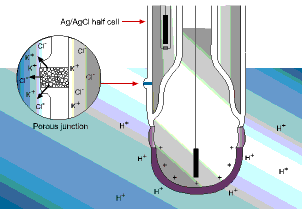
The reference electrode is comprised of three key components, a silver/silver chloride (Ag/AgCl) half-cell, electrolyte (KCl), and a porous junction. The reference electrode is considered the weak link in the measurement chain. In fact, statistical analysis has shown that 80% of pH sensor failures can be attributed to a failure of the reference electrode. The primary reason for failure is contamination.
The junction of the reference electrode connects the Ag/AgCl half-cell to the measuring electrode via the process solution. This direct electrical connection is necessary for the entire electrode to function. This connection is traditionally made with a salt solution (KCl) through a porous junction.
Conventional reference failure modes are:
* Premature electrolyte (KCl) depletion.
* Dilution of electrolyte by the process.
* Contamination of electrolyte.
* Chemical reactions with the Ag/AgCl half-cell.
* Clogging or coating of the porous junction.
Various developments of the reference have tried to address these problems by using different constructions such as gelled KCI, multiple liquid junctions, different porous junction materials and flowing or pressurised KCl reservoirs, with differing degrees of success. Broadley James Corporation has developed a sensor specifically for process applications that incorporates a patented reference electrode. The DynaProbe pH sensor design addresses the problems of premature failure in the following ways.
* A patented solid-state reference half-cell with immobilised electrolyte that minimises electrolyte depletion or dilution.
* The liquid junction, fabricated from a single core and saturated with conductive electrolyte, reduces cogging and coating of the reference.
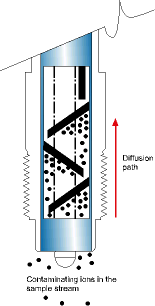
* A unique Ion Trap, which holds contaminating ions that migrate from the sample stream and naturally diffuse into the reference electrode. They are retained in the trap areas while still permitting a contiguous conductive path necessary for the reference cell function.
* The sensor is moulded in a single chemically resistant polymer, which reduces the number of wetted materials, eliminated O-ring seals and simplifies maintenance.
The combination of these features produces a pH sensor with a high temperature and pressure specification and a good resistance to contaminating ions. The DynaProbe has been successfully applied in many different process applications where conventional sensors have failed prematurely, including sugar processing, paper and pulp, waste water treatment and dyeing.
The pH sensor can be supplied with a variety of different temperature sensors for automatic temperature compensation, which provides universal compatibility with all pH and ORP (redox) instruments. Different accessories, materials and mounting options enable the DynaProbe to be easily applied to a variety of different situations and to all types of pH instruments, making it very cost effective to upgrade existing installations.
LTH and Broadley James Corporation are represented in South Africa by Temperature Controls.
For more information contact Andy Brown, Temperature Controls, 011 791 6000, [email protected], www.tempcon.co.za
| Tel: | +27 11 791 6000 |
| Email: | [email protected] |
| www: | www.tempcon.co.za |
| Articles: | More information and articles about Temperature Controls |

© Technews Publishing (Pty) Ltd | All Rights Reserved