
The treatment of wastewater, especially sewage, has recently become more critical as rural communities move into the cities, placing unprecedented pressure on sewage treatment plants.
This treatment has a number of clearly defined stages: filtration, primary clarifiers, pre-treatment, aeration, secondary clarifiers, thickeners, primary and secondary digesters, etc.
Some of these stages are mechanical or physical processes, but the most complex are chemical or biological reactions. Important parameters that need to be measured are ammonium, phosphates, nitrates and nitrites. When treating sewage, it is desirable to remove nitrogen as this helps the biological cleaning process. A common practice is to use the natural process of nitrification first, and then denitrification. Phosphorous is the main fertiliser apart from oxygen that causes water eutrophication.
The growth of algae diminishes only when the phosphorous concentration falls below 1 mg/litre. A by-product of denitrification in a biological clarifier is dissolved ammonia, which must be monitored carefully. More often, the reduction of energy costs is the main reason for using ammonium measurement in the aeration process. Reliable and accurate measurement of these parameters is essential for sewage treatment optimisation and cost-effective operation.
Staiger Mohilo analyser philosophy
A new generation of analyser measuring systems for sewage and wastewater treatment plants has been developed by Staiger Mohilo, recently acquired by Endress+Hauser. It combines the repeatable availability of concentration values with high efficiency due to low consumption of chemicals and minimum wearing parts in a quasicontinuous procedure. Using colorimetrical measuring techniques, which involve the addition of reagents to the process sample, has many advantages. The intensity of the colour indicates the concentration of each substance: ammonia (NH4), nitrate (NO3), phosphate (PO4) and nitrite. To detect the colouration, the coloured sample is illuminated and the light intensity is measured (Figure 1). The light intensity is only attenuated at a specific wavelength, so it is possible to use a single device at different wavelengths to determine the concentration of the different chemicals.
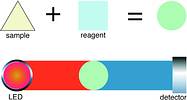
Photometrical processes normally have relatively small known transverse sensitivities as a result of a specific reaction causing specific colour changes which are measured at specific wavelengths.
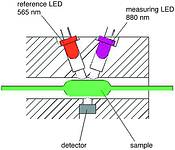
Measuring procedures are based on DIN to allow recourse to tried and trusted procedures with known influence factors, the only exception being the determination of the nitrate ion. For ammonium, the DIN 38406-5 (sodium dichloro isocyanrate and sodium salicylate) specification measures the quantitative colouration in a photometer at a wavelength of 660 nm. By using optimised reagent concentrations, and careful design, a reliable result can be obtained within three minutes. For nitrates, the analyser operates differently from DIN 38405-9 in order to avoid development of toxic compounds and favours chromotropic and sulphuric acid. The colouration caused by the nitrate is quantitatively measured in a photometer at a wavelength of 430 nm. Results are available within 6 min. In the case of orthophosphate, the very economical principle of the molybdate-vandate method proposed in the notice ATV-M 269 is used. The colouration is measured quantitatively in a photometer at a wavelength of 880 or 430 nm. In all cases the measuring light is compared in the photometer with a reference light at a wavelength of 565 nm to prevent any falsification of the measurement due to turbidity.

For potable water and industrial applications, the analysers are provided for nitrite, iron, calcium, sulphite and fluoride with additional parameters being prepared.

| Tel: | +27 11 262 8000 |
| Email: | [email protected] |
| www: | www.endress.com |
| Articles: | More information and articles about Endress+Hauser South Africa |
© Technews Publishing (Pty) Ltd | All Rights Reserved